Health + Medicine
Feature
New Israeli Made Immunotherapy Targets Myeloma
Elisheva Milo is a 73-year-old retired industrial designer from Haifa. Descended from a family that has lived in the Land of Israel continuously for over 2,000 years, she is a devoted aunt and great aunt.
Uri Lahav, 49, is an occupational therapist from Jerusalem. Husband to Karni and father to three children ages 15 to 20, he is the son of parents who immigrated to Israel from the United States about 50 years ago.
Despite their very different backgrounds, Milo and Lahav share something critical: This past year, both ran out of options in their battles against multiple myeloma, a cancer that relentlessly destroys bone marrow. For years—13 for Milo, seven for Lahav—they moved from one therapy to the next, until there was nothing left to try. Both are among the first group of 57 seriously ill Israeli patients who readily agreed to take part in the trial of an experimental made-in-Israel immunotherapy treatment.
The outcome of three years’ work by physicians and scientists at the Hadassah Medical Organization and Bar-Ilan University, the trial’s interim results are “outstanding,” said Dr. Polina Stepensky, head of HMO’s bone marrow transplantation and cancer immunotherapy department. Ninety percent of myeloma patients in the trial have seen improvement so far, and over half—57 percent—have gone into remission. Patients in the trial were between the ages of 44 and 84, and there has been no evidence that age affected successful outcomes.
“With Israel the startup nation, it had been clear to me that we should and could find an answer for the growing numbers of people with incurable multiple myeloma,” said Dr. Stepensky. A hematologist-oncologist, pediatrician and stem cell transplantation expert, she is co-inventor of this new Israeli immunotherapy—a treatment that uses the immune system to battle disease—for multiple myeloma. Incidence of the illness, the second most common blood cancer, is steadily growing for apparently unexplained reasons. This year, an estimated 176,000 people worldwide—550 of them in Israel, 35,730 in the United States—will be diagnosed with this disease, which causes blood cells manufactured in the spongy bone marrow to be crowded out by fast-proliferating cancer cells.

“When I graduated from Hadassah Medical School in 1998, median survival for multiple myeloma patients was less than three years,” said Dr. Stepensky. “With new medications and bone marrow transplantation, we’ve stretched this to over a decade. But in the end, all existing therapies lose effectiveness. We needed another approach.”
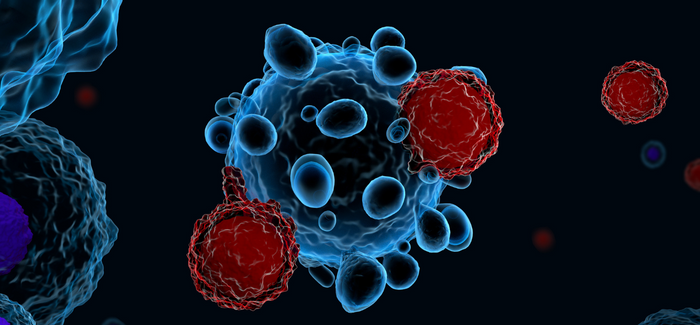
That other approach, called chimeric antigen receptor (CAR) T-cell therapy, circumvents the slash-and-burn of conventional cancer treatments—surgery, chemotherapy and radiotherapy and their brutal side effects—and weaponizes the patient’s own immune system. After seeing the work of immunologist and genetic engineer Dr. Michel Sadelain at the Memorial Sloan Kettering Cancer Center in New York in 2017, Dr. Stepensky convinced Hadassah that Israel could manufacture and develop this treatment, too.
She brought on board a Bar-Ilan University team led by renowned French-born immunologist Cyrille J. Cohen, head of the university’s Laboratory of Tumor Immunology and Immunotherapy. Their role was to create a synthetic cellular molecule that instructs the patient’s immune system T-cells which cancer cells to target.
“It took many different designs until we identified the optimum molecule, but we got there,” said Cohen.
The Israeli CAR T-cells are formally classified as HBI0101—the HBI referencing its creators, Hadassah and Bar-Ilan—and use the same general approach as CAR T-cells engineered in the United States and China, which lead the world in CAR T-cell therapy trials and applications. In America, where researchers have focused on molecules and vectors different from the Israeli team’s research, CAR T-cell therapy was approved by the Food and Drug Administration six years ago, though it is still considered experimental.
READ MORE: Meet Suzanne Patt Benvenisti, Hadassah’s New Head In Israel
The treatment is also expensive, costing hundreds of thousands of dollars. In China, its cost is lower, but regulatory standards there differ radically from the West. As Dr. Stepensky noted, the potentially far more modest Hadassah price tag makes Israel’s CAR T-cell therapy accessible to the estimated 120 Israelis needing it at any one time as well as to patients from outside Israel. She expects it to be in mainstream use within five years.
Proof of concept and preclinical studies of the CAR T-cells were the task of Hadassah, which “raised millions of donor dollars to take this technology through its crucial initial stages,” said Dr. Yoram Weiss, HMO’s director general. Those steps included “building Good Manufacturing Practice-graded master cell banks and manufacturing facilities, ones that use globally recognized production guidelines.”
In 2020, within a record-breaking three years, Israel’s Health Ministry approved clinical trials of the Hadassah-Bar-Ilan CAR T-cell therapy.
It was in October of last year, after Uri Lahav learned he had reached the end of the conventional treatment road, that doctors proposed the new treatment.
“I’d heard of it as something promising but not yet available in Israel,” recalled Lahav, whose mother, Elisheva, volunteers at Hadassah Hospital Mount Scopus. “When Hadassah suggested it, I thought: ‘Wow! It’s here in Israel!’ And leapt at it.”
For Lahav, Milo and other participants, the trial, which took place in Hadassah’s bone marrow transplantation and cancer immunotherapy department, began with collecting the patient’s T-cells. A catheter in one arm drew out blood and passed it through a gently pinging plasmapheresis machine, which extracts plasma and the immune system cells it contains and then returns the remainder of the blood. Next came three days of light chemotherapy to ease introduction of the enhanced cells.
Meanwhile, in Hadassah’s specially built lab, researchers isolated the T-cells, enhanced them with the artificial molecules developed by the Bar-Ilan team and replicated them. To the delight of all, when the enhanced T-cells were infused back into the patient, the cells did exactly as programmed. They hunted down and destroyed the multiple myeloma cells.
“It’s only a short time since the treatment, so too early for results,” said Lahav, “but my energy is returning. I feel good and I’m very hopeful.”
For Milo, too, joining the trial was “an easy decision because I had nowhere else to go. It wasn’t too hard a treatment, easier than my two bone marrow transplants,” she said. “I spent two weeks in hospital in isolation, then came back regularly for several weeks for follow-up. Ten months later, I’m still in remission and feel good.”
By January 2023, the results of Hadassah’s clinical trial were making medical headlines. The Los Angeles-based Immix BioPharma proposed a collaboration. Under the research and licensing agreement signed between Immix and the technology transfer companies of Hadassah and Bar-Ilan—Hadasit and BIRAD—next-generation Israeli CAR T-cell therapy will be developed and commercialized, and its production extended to the United States.

“With Immix support, we plan greater automation and optimization of our cell production and continuation of our clinical studies,” said Dr. Stepensky. “We’re also waiting for Israeli Health Ministry authorization for frozen cell production as well as fresh, which will allow us to take more people into our trial. At the moment, we can handle only one new patient a week, and our waiting list is six months long.”
The therapy, she pointed out, is still in its early stages. While Hadassah’s CAR T-cell therapy puts multiple myeloma patients into remission of unknown duration, it does not cure them. Dr. Stepensky anticipates, however, that repeated CAR T-cell therapy treatment will convert the cancer from a fatal to a chronic disease. And she looks to the world of patients beyond those with multiple myeloma.
“We’ll be evaluating new targets and technologies,” she said. “Other blood cancers, such as leukemia and lymphoma, are an obvious first step, perhaps followed by solid tumors.
“I believe that this type of personalized cell therapy is the future,” added Dr. Stepensky. “I dream of a facility here at Hadassah that develops individualized cellular treatments for children and adults, for different cancers and for all other diseases where gene engineering can play a critical role.”
Wendy Elliman is a British-born science writer who has lived in Israel for more than four decades.










 Facebook
Facebook Instagram
Instagram Twitter
Twitter
Lisa Kaye says
Thank you for this most encouraging article. Leave it to Hadassah to get things DONE !
Thank you Wendy for your informational and educational report.
I myself am a 4 time cancer survivor but not of this nature.
I am writing to you today with a dual mission.
My beshert who has multiple myeloma has tried a stem cell transplant and many other immune therapies none of which have worked. Kyropolis and dura combined intravenously and venetoclax orally finally stoped working.
Here’s the other portion of the dream. Besides remission, we are truly interested in philanthropic efforts where we can make a difference to this population who puts on their warrior suits daily to fight the cancer that keeps on fighting. We want change and a life to share our success and generosity with. Please let me know your thoughts on the best path to achieve our goals
LAURENT RAHMINE says
Hello , my mother had the Myelome , may i have the phone number of Mendy .
I am Avraham Rahamine my phone number is +972 546486784
Thanks a lot
màn hình led says
The article highlights the inspiring story of Elisheva Milo and Uri Lahav, who both battled multiple myeloma and participated in an experimental immunotherapy trial in Israel. The preliminary results of the trial are described as outstanding, with a high percentage of patients experiencing improvement and remission, irrespective of age. This demonstrates the potential of Israeli medical innovation in the fight against multiple myeloma, a challenging and growing health concern globally.